
| Home |
| Drug Mix-Ups Threaten Patient Safety |
When he returned to see his family physician 2 months later, DK brought along his medication bottles as requested; the doctor realized the mistake and contacted the pharmacy. Fortunately, the patient did not suffer any immediate harm other than not benefiting from improved control of his blood glucose levels. But he did not receive the medication he needed to achieve normal blood glucose levels for an extended time and instead received unneeded antibiotics.
As children, we learned that the combination ice cream with strawberry, vanilla, and chocolate is called "Neapolitan," not "Napoleon." Because these words have phonetic (sound-alike) and orthographic (look-alike) qualities, confusion is understandable. Similarly, medication names can be confused due to similar looking or sounding qualities.
Health professionals who prescribe, dispense, or administer medications have likely encountered medication errors involving look-alike or sound-alike (LASA) drug name pairs. While confusing a name of ice cream is harmless, adverse drug events that occur due to LASA qualities are frequent and potentially dangerous.
The osteoporosis agent Evista (raloxifene) has been dispensed instead of Avinza (extended release morphine) due to both look-alike and sound-alike qualities.[1] While it may seem unlikely, a prescription for the antidiabetic agent Avandia (rosiglitazone) may be mistaken for the anticoagulant Coumadin (warfarin) due to look-alike tendencies when handwritten in cursive[2] (Figure 1).
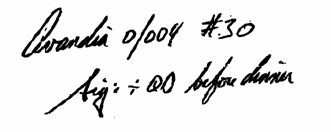
Figure 1. Handwritten "Avandia" can look surprisingly like "Coumadin."
LASA medication errors occur when drug products that look alike when written or sound alike when spoken are confused for one another. In addition to the examples provided above, LASA qualities have led to the mix-up of Amaryl (glimepiride), an antidiabetic agent, for Reminyl (galantamine), an Alzheimer's agent, resulting in severe hypoglycemia.[3] Because of this confusion, Reminyl has since been renamed Razadyne.
Drug name similarities can exist between brand names (eg, Diovan and Darvon), between generic names (eg, cisplatin and carboplatin), or between a brand and a generic medication (eg, Hespan and heparin).[4]
Drug name confusion often occurs as new pharmaceuticals are brought to market. Although the number of new drugs approved each year is relatively small -- 22 in 2006, for example[5] -- these agents join thousands of over-the-counter, prescription, and biologic agents already in use. In an effort to reduce confusion between brand names, the pharmaceutical industry has adopted various name testing protocols that incorporate practitioner testing and computer analysis.[6] The US Food and Drug Administration (FDA) rigorously evaluates about 400 proprietary drug names annually and rejects about 1/3 of them.[7]
The
USP-ISMP Medication Errors Reporting Program, operated by the US
Pharmacopeia (USP) in cooperation with the Institute for Safe
Medication Practices (ISMP), collects and analyzes voluntary error
reports
Due to the frequency of drug name mix-ups, the ISMP has compiled a list of medications involved in potential or actual errors due to LASA confusion.[8] This list is available for use as a tool to educate health organizations, prescribers, and other healthcare professionals about LASA drug name pairs.
Table 1 lists the top 10 most common drug interchange errors caused by LASA qualities, as reported to the Pennsylvania Patient Safety Reporting System (Pa-PSRS).[9] Of these top 10 errors, 8 involve narcotics, 1 involves a benzodiazepine, and 1 involves insulin. Not only are these errors common, they involve "high alert" medications (those that have a high likelihood of causing harm when used incorrectly). The fact that 8 of the 10 name pairs include generic drug names illustrates how both product nomenclature and the trade-to-generic name continuum regularly give rise to mix-ups.
Table 1. Top 10 Look-alike, Sound-alike Drug Name Pairs[9]
| Morphine/ hydromorphone |
| HYDROcodone with acetaminophen/ OXYcodone with acetaminophen |
| Oxycodone/Oxycontin |
| Alprazolam/lorazepam |
| Acetaminophen with codeine/ OXYcodone with acetaminophen |
| OXYcodone/ OXYcodone with acetaminophen |
| MS Contin/Oxycontin |
| Novolog Mix 70/30/Novolin 70/30 |
| Morphine/meperidine |
| Propoxyphene with acetaminophen/OXYcodone with acetaminophen |
The
USP maintains a national database, MEDMARX, that is used by hospitals
and other healthcare organizations to report, track, and analyze
medication errors. The eighth annual MEDMARX data report, released in
January 2008, concluded that 1470 commonly used drugs are involved in
errors caused by LASA name confusion.[10] Errors included in
that study were reported from 2003 to 2006 and covered prescription
medications, over-the-counter medications, and dietary supplements.
More than 3000 possible drug name confusions were noted.
All of the 10 most prescribed drugs have been implicated in drug name pair confusion, and such adverse events have the potential to cause significant patient harm.[10] In fact, each time a LASA error reaches a patient, a 2-pronged consequence results: an error of omission occurs because the indicated pharmaceutical agent is not received, and an error of commission occurs when a non-indicated agent is received. In the opening patient case, for example, the patient did not receive metformin, a therapy needed to manage his diabetes (an error of omission), but instead received an unneeded antibiotic (an error of commission).
Possible consequences of LASA medication errors include lack of therapeutic response, acute drug reaction, disability, or death. The eighth annual USP MEDMARX Data Report showed that 1.4% of reported LASA medication errors resulted in patient harm, with some causing or contributing to death. The actual incidence is presumed to be higher.[10] Table 2 lists the drug name pairs most commonly associated with patient harm. Each name pair appears on at least 1 roster of problematic drug name pairs maintained by USP-ISMP, Pa-PSRS, or The Joint Commission (TJC). The top 10 drugs involved in harmful medication errors are insulin, morphine, heparin, hydromorphone, warfarin, fentanyl, potassium chloride, vancomycin, enoxaparin, diltiazem.[10]
Table 2. Examples of Name Confusion Among Drugs Most Commonly Involved in Patient Harm
| Drug Category | Product |
|---|---|
| Antithrombotics | Warfarin (Coumadin vs Avandia) Heparin vs Hespan |
| Insulins | Lente vs Lantus Humalog vs Humulin Humalog mix 75/25 vs Humulin 70/30 Humalog vs Humulin R Humalog vs regular insulin Novolin 70/30 vs Novolog Mix 70/30 Novolog vs regular insulin Novolog vs Novolin R |
| Opiates | Morphine (Astramorph, Duramorph, Infumorph, etc) vs hydromorphone Fentanyl vs sufentanil Concentrated oral liquid morphine (Roxanol) vs conventional Concentration oral liquid morphine |
The National Coordinating Council for Medication Error Prevention and Reporting (NCCMERP) recommends that telephone and verbal orders be used only in urgent situations when use of electronic or written orders is not possible. NCCMERP recommends that verbal or telephone orders never be given for chemotherapy.[11]
Some medication pairs are especially error-prone due to sound-alike qualities. For example, Neurontin and Noroxin sound similar, as do diphenhydramine and dimenhydrinate. Thus, good communication is essential to prevent LASA medication errors. Specific behaviors -- such as reading back transcribed drug information and having the prescriber verify accuracy -- are valuable because they add redundancy when high-stakes information is communicated.[12]
To prevent drug name pair confusion, institutions should develop written policies that detail expectations of individuals who give and accept telephone orders.[13] Policies should include both operational goals (eg, clinicians will transmit and receive verbal and telephone orders accurately and only when absolutely necessary) and behavioral expectations (eg, receiver must write down the order and then read it back to the prescriber, confirming spelling of the drug name, dose, and indication).[6] This process ensures not only that the order has been heard correctly, but also that it has been transcribed correctly. To protect patients, prescribers should insist upon "read back" and allow an appropriate amount of time for it.
Prescriptions that are called to community pharmacies frequently default to a voice mail system or interactive voice response device. This eliminates the possibility of a read-back and makes this mode of communication more error-prone. When leaving prescription data on voice recording devices, prescribers and others who call in prescription data must enunciate clearly, take time to spell drug names, and use single digits to state numbers (eg, "five-zero" instead of "fifty" lessens the chance that "fifty" will be misheard as "fifteen").
Any strategy incorporating redundancy into the handwritten communication process is desirable. Including both the brand and generic name or adding the purpose for drug use on written prescriptions or medication administration records may prevent LASA errors from being set in motion. These redundancies also help clinicians recognize and take corrective action before errors reach a patient. Handwritten medication orders should be clearly printed in block letters.[7] This includes prescriptions, medication orders, and data transcribed onto the medication administration record.
Table 3 lists communication strategies that are recommended for healthcare professionals to minimize the risk of LASA drug confusion.
Table 3. Communication Strategies for LASA Error Prevention[12,13]
For Written Orders
|
For Verbal Orders
|
Many medications are packaged in bottles with similar shapes and similar labels, making it easy to confuse one drug with another.

Figure 2. Similar looking drug bottles are easily mixed up.
Changing a product's name or appearance may help prevent LASA medication errors. For example, "tall man" letters call attention to a drug's name and distinguish it from its LASA name pair. Using tall man lettering, diphenhydramine would be presented as diPHENhydrAMINE, whereas dimenhydrinate would be diMENhyDRINATE. The unique letter characters in look-alike drug name pairs may also be highlighted using color, reverse color background, italics, underline, and other distinguishing depictions.
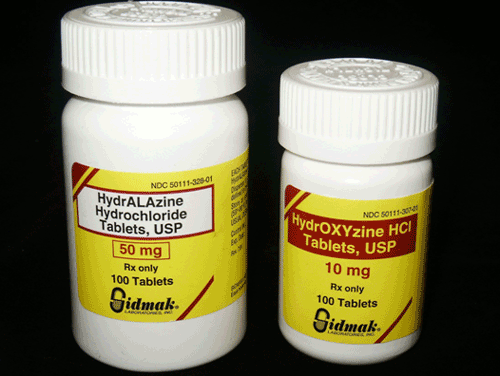
Figure 3. "Tall man" letters and reverse color background help distinguish one drug from another.
At ISMP's suggestion, the FDA has compiled a list of look-alike drug name pairs in which tall man lettering is recommended.[17] With FDA approval, that practice has been adopted by a number of manufacturers to differentiate look-alike brand names when medication errors were recognized post-market. For example, Eli Lilly uses reverse-color background, italics, and tall man lettering to help differentiate Zyprexa from Zyrtec.
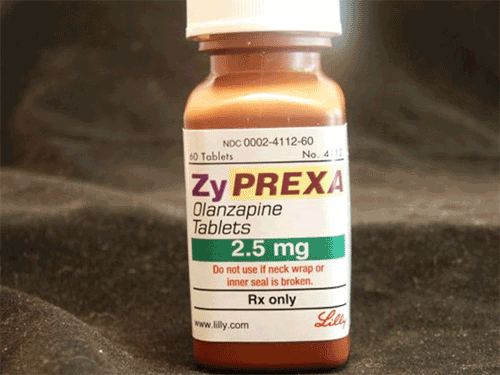
Figure 4. Italics, "tall man" letters used on bottle of Zyprexa.
The metformin-metronidazole mix-up described in the first case study originated when the wrong agent was selected from a product list on the pharmacy computer screen. But computer systems may be valuable assets for preventing LASA mix-ups because they can apply distinguishing features to portions of confused drug name pairs in order to call attention to them.[6] Once adopted, differentiation strategies should be extended to computer-generated medication administration records and automatic dispensing cabinets whenever feasible. A related strategy is to ensure that LASA drug name pairs do not appear consecutively on computer screens or product selection lists.
Changes in manufacturer labeling or overwrap may be necessary to help prevent LASA errors. For example, different intravenous medications may resemble one another due to similar sizes, labels, and overwraps. These similarities have contributed to a host of adverse drug events.[18] Warnings should be affixed to labels or overwraps to alert practitioners to the potential for LASA confusion.
Storage strategies can be implemented to reduce confusion as well. Drugs with similar looking or sounding names should not be stored next to one another.[7] Medication storage bins in pharmacies, nursing units, and other storage areas should have warning labels cautioning healthcare professionals about the potential for drug name mix-ups.[18]
Technology is available to assist with error reduction at every point of the drug delivery process. Electronic prescribing (eg, computerized physician order entry [CPOE]) virtually eliminates handwritten prescriptions. Pharmacy robotic systems that use barcode technology can dispense medications in both ambulatory and inpatient settings, reducing the risk of human error. Medications checked by barcode scanners on automated dispensing cabinets and at the point of care can reduce the risk of error at the time of administration.
Integrating computers into the prescribing process provides many benefits. Computer drug databases can alert prescribers to possible LASA confusion, especially when order entry systems interface with clinical data. In addition, the computer can warn clinicians who enter orders about potential look-alike or sound-alike errors involving the drugs they are listing. Patient-specific drug and disease precautions can be included in drug information sheets provided to patients by community pharmacists along with dispensed prescriptions.[19]
Barcode technology can help circumvent or validate steps in the medication use system that rely on human interpretation and are therefore subject to human error. The implementation of barcode technology, along with electronic medication administration records, was shown to reduce medication administration errors by 54%.[20] ISMP supports a recommendation to establish a Joint Commission National Patient Safety Goal that would require institutions to acquire this technology or prepare for future installation.
As discussed earlier, communication between the prescriber and the dispensing pharmacist is key to preventing LASA errors, especially a "read back" approach that verifies the correct information. When sending written orders to a pharmacy, prescribers should add the prescription's purpose; this helps the pharmacist identify the intended drug when handwriting is poor, for example, or when a faxed prescription contains extraneous marks.[13] Clinicians who dispense and administer medications should match the purpose of the medication to the patient's condition, to verify the suitability of the medication.
Whenever feasible, patients should be educated and engaged in their medication use. Healthcare professionals, especially community pharmacists, should show medications to patients prior to dispensing or administering them to verify that the drug name, appearance, and purpose are all correct.
By teaching patients what to expect about the medication they are about to receive, prescribers enable the patient to recognize errors that may occur. For example, a patient expecting to receive Celebrex for pain would likely question the receipt of Celexa for depression. Counseling from a pharmacist can also help people take their medications safely and effectively. In acute-care and long-term care settings where clinicians administer drugs, education helps individuals understand their plan of care. But in the case of LASA agents, the act of educating a patient is even more important because it provides a final opportunity to double-check the accuracy of an error-prone drug before releasing it to the patient.
Patients should be advised that LASA medication errors can occur, and that they can play a role in preventing these errors. Table 4 lists strategies that patients should use. Clinicians are encouraged to provide this list to patients to help them learn to be effective advocates. Patients should be encouraged to keep written or electronic medication logs. In addition to other safety benefits, written medication lists help patients become familiar with their medications, including generic names. This familiarity helps them identify LASA mix-ups should they occur.
Table 4. Consumer Measures for LASA Error Prevention[1,7,12,23]
| Be aware that mix-ups with medications that look alike or sound alike (LASA) are not uncommon |
| Know what medications you are taking and why |
| Make sure your doctor tells you the purpose for each medication and writes it on the prescription itself |
| Know the name and strength of a prescription before leaving prescriber's office |
| When you pick up a prescription, always speak to the pharmacist to review the medication and directions for taking it |
| Make sure the pharmacist mentions the same purpose the doctor mentioned to assure that the right drug has been dispensed |
| Know how to take medications and understand directions |
| Ask questions, if in doubt, and persist with questioning until you are certain you are receiving the correct drug |
| Keep a list of medications you take, including dietary supplements and over-the-counter medications. |
| Update the medication list whenever a change occurs |
| Give an updated copy of your medication list to all healthcare providers at every visit |
| Learn generic drug names as key identifiers |
| Tell all practitioners who care for you about changes in your health |
| Ensure that refilled prescriptions contain |
| Ask for written information about prescribed medications |
| Contact health professionals if a LASA error is suspected |
Patients
should be encouraged to ask the prescriber, a pharmacist, or a nurse if
they have any questions about a medication they are taking. Healthcare
professionals should give patients positive reinforcement, as well as
clinical information, whenever patients seek clarification or
additional information.[21] Table 5 lists Web sites that
consumers may visit for additional medication safety information; this
list may also be provided to patients as a resource.
Table 5. Medication Safety Web sites for Consumers
| Institute for Safe Medication Practices | www.ismp.org |
| Food and Drug Administration | www.fda.gov |
| United States Pharmacopeia | www.usp.org |
| Agency for Healthcare Research and Quality | www.ahrq.gov |
| National Coordinating Council for Medication Error Reporting and Prevention | www.nccmerp.org/consumerinfo.html |
| BeMedwise | www.bemedwise.org |
| National Council on Patient Information and Safety | www.talkaboutrx.org |
| Safemedication.com | www.safemedication.com |
For patients to understand that they are an integral part of their medication safety team, they should be treated as valued team members. Knowledge of the patient's health literacy level and cultural beliefs are needed to teach effectively and promote adherence to a recommended course of therapy.[22]
Education should start at the site of diagnosis when prescribing occurs. Prescribers should tell patients the drug name, dose, and purpose, instruct them about how to take the drug, and teach them about the expected effects and any predictable side effects. Knowing what to expect from drug therapy helps patients recognize and report unexpected effects that may occur during normal drug therapy and almost certainly will occur if the wrong medication is received.
Validating patient understanding of prescribed therapies, including drug regimens, has been identified as a vital component of efficacious healthcare. One easy-to-adopt technique involves patient "teach-back," in which patients demonstrate mastery of content by teaching it back to the provider.[21,23] The National Coordinating Council for Medication Error and Prevention also provides recommendations to foster effective verbal communication.[11]
Any time a new drug is first prescribed, clinicians should consider the potential for drug-drug interactions, duplication of therapy, and LASA name confusion. In addition, they should review safety information sources very frequently for information regarding LASA drug confusion. Clinicians should take a proactive approach to reducing the risk of LASA errors.
In particular, prescribers should familiarize themselves with the most common LASA medication errors associated with drugs they prescribe frequently. In doing so, they may be able to circumvent errors in their own practices.
Table 6 lists Web sites that are helpful resources for clinicians. In addition, ISMP publishes newsletters and quarterly action agendas that highlight problematic drug name pairs. These publications also offer clinicians tools and practical risk-reduction strategies for preventing LASA errors.
Table 6. Organizations Providing Drug Safety Information[18]
| Agency for Healthcare Research and Quality | www.ahrq.gov |
| American Pharmacists Association | www.aphanet.org |
| American Society of Health-System Pharmacists | www.ashp.org |
| Food and Drug Administration | www.fda.gov |
| Institute for Safe Medication Practices | www.ismp.org |
| Institute of Medicine | www.iom.edu |
| The Joint Commission | www.jointcommission.org |
| National Coordinating Council on Medication Error and Prevention | www.nccmerp.org |
| National Patient Safety Foundation | www.npsf.org |
| United States Pharmacopeia | www.usp.gov |
Voluntary adverse event reports often lead to corrective actions such as widespread dissemination of hazard alerts by ISMP, FDA, and manufacturers, and even changes in drug names. For example, after several deaths were reported following mix-ups between amrinone and amiodarone, ISMP petitioned United States Adopted Names to change the proper name, amrinone, to inamrinone. This has effectively eliminated the problem name pair along with associated deaths.
Medication error reports show how people -- both clinicians and patients -- interact with drugs in ways that cannot be readily predicted during clinical trials.
Internal error reporting can help identify error-prone conditions in an organization's medication use system. The most robust internal error reporting programs include patients and family members, as consumers often catch errors and near-misses that were not perceived by professionals.[21] Once system deficits are identified, proactive measures such as auxiliary labeling, drug storage changes, or clinician education may be undertaken to remedy these conditions and prevent similar mishaps in the future.
Three organizations administer national voluntary reporting programs that allow individuals and organizations to report medication errors in confidence (Table 7). In fact, ISMP, USP, and the FDA share medication error reports, creating a valuable bank of aggregate information about errors and error-prone conditions.
Any report sent to the USP-ISMP Medication Errors Reporting Program is automatically shared with the FDA; thus, all 3 organizations can be alerted with a single report. This also offers the greatest opportunity to initiate widespread educational efforts and national alerts by all 3 groups through their Web sites and newsletters. In addition, this facilitates advocacy efforts by ISMP to best assure that important issues are being addressed by industry and regulatory authorities and that healthcare practitioners and organizations are rapidly notified through published newsletters and alerts.
Table 7. Error-reporting Links
| USP-ISMP Medication Errors Reporting Program | www.ismp.org/orderforms/reporterrortoISMP.asp or www.usp.org/hqi/patientsafety/mer/ |
| US Food and Drug Administration MedWatch | www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm |
Other name changes that were prompted by drug error reports include Omacor (changed to Lovaza) and amrinone (changed to inamrinone, as noted above). Seldane, however, was taken off the market due to cardiac concerns, and Clarinex is the name of an altered formulation of Claritin.
Reports from frontline clinicians are especially important when errors involve LASA products because high-level error reduction strategies -- such as drug name changes or packaging alterations -- are sometimes needed to prevent future errors. Product problems may occur with a frequency that should constitute a "call to action," but the incidence or significance of those problems may be lost within the construct of individual practice settings.
ISMP has advocated for such changes with the FDA and manufacturers, and it was instrumental in changes such as Levoxine being renamed Levoxyl, Omacor becoming Lovaza, and amrinone becoming inamrinone. ISMP's advocacy yielded results because it was based on aggregate data pulled from a rich, detailed, and credible database of voluntary error reports. Thus, reports from frontline clinicians enable this advocacy to occur.
As a condition of accreditation, TJC requires that organizations study the potential for LASA errors to occur in their setting and take proactive measures to prevent these errors. Once potentially problematic LASA name pairs are identified, organizations must create an institution-specific list and take actions to avoid interchange of these medications. Progress must be evaluated and the list formally reviewed at least annually.[25]
Another TJC requirement for accreditation of health organizations is to create and maintain a formulary of available medications.[13] Those involved with formulary maintenance should consider the possibility of drug name confusion when adding any medication to the formulary.
In addition to formulary review, medication administration policies and procedures should be reviewed to ensure that LASA error avoidance procedures have been implemented.[13] Known error-prone LASA pairs should be publicized within organizations. Near-misses that clinicians have encountered within the organization should be reported and publicized. Publications that report problematic LASA drug name pairs should be reviewed frequently, and the information passed on to frontline clinicians regularly.
Organizations that have not yet done so should have plans to implement electronic prescribing. If electronic prescribing is unavailable, preprinted orders and prescriptions should be used whenever feasible.[6]
Medication errors due to look-alike, sound-alike qualities are common and potentially dangerous. Health professionals should be aware of this danger and implement strategies to prevent error occurrence. LASA prevention strategies often overlap with general "good practice" recommendations for reducing all medication errors. Table 8 summarizes error-reduction tools and strategies that are available for all healthcare professionals.
Table 8. Health Professional Strategies for LASA Error Prevention[6,11,15]
| Do not store LASA drugs alphabetically. Store out of order or in separate places (eg, fast mover section). |
| Prescriptions should include all necessary elements including drug name, strength, dosage form, frequency, etc. |
| Match prescription indication to patient's medical condition before dispensing or administering. |
| Implement drug-specific strategies for known error-causing pairs (eg, stock different strengths). |
| Report actual and potential errors. |
| Discuss potential causes of errors and strategies to remedy. |
| Open medication in front of the patient in order to confirm expected appearance and review indication. |
Furthermore, patients should be educated and involved in LASA drug error prevention. Goals of voluntary reporting include: dissemination of information about errors -- including those rooted in look-alike, sound-alike similarities -- and leveraging the information to build safer medication use systems. LASA drug identification and error prevention are priorities for patients and a wide array of professional stakeholders.