
| Home |
| New Guidelines for Managing Hypercholesterolemia |
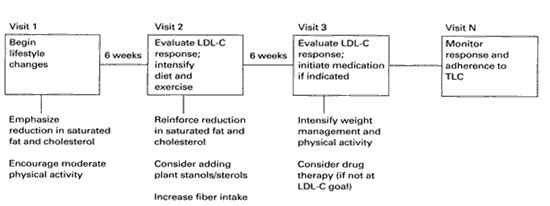
Figure 1. Steps in Implementing Therapeutic Lifestyle Changes (TLC)
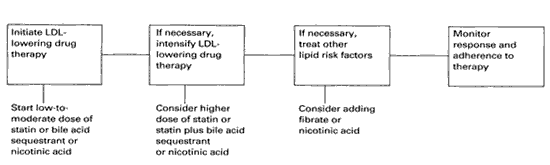
Figure 2. Steps in Initiating Lipid-Modifying Drug Therapy
| A 10-year estimate of absolute CHD risk using Framingham data is provided
for use in patients with |
| Patients with diabetes and persons with multiple risk factors plus a 10-year risk for CHD of > 20% are identified as CHD risk equivalents and targeted for more intense treatment. |
| Identified an LDL-C level of < 100 mg/dL as optimal. |
| Recommended greater restriction of saturated fats and cholesterol and use of dietary adjuncts (i.e., plant stanols/sterols and viscous fiber) as "therapeutic lifestyle changes" (TLC). |
| Provided a way to identify patients with the metabolic syndrome and recommended intensified TLC for these patients. |
| Recommended non-HDL-C goals for patients with triglycerides > 200 mg/dL after LDL-C goal has been achieved. |
| Lowered the triglyceride classification. |
| Raised the definition of low HDL-C to < 40 mg/dL. |
| Presents strategies to promote adherence with lipid-modifying therapies. |
| Places emphasis on long-term prevention. |
| Classification | LDL-C Level (mg/dL) |
|---|---|
| Optimal | < 100 |
| Above optimal | 100-129 |
| Borderline high | 130-159 |
| High | 160-189 |
| Very high |
| Classification | Triglyceride level (mg/dL) |
|---|---|
| Normal | < 150 |
| Borderline high | 150-199 |
| High | 200-499 |
| Very high |
| Risk Category | LDL-C Goal |
|---|---|
| CHD and CHD risk equivalent | |
| Multiple ( |
< 130 mg/dL |
| 0-1 risk factor | < 160 mg/dL |
| Age (men |
| Family history of premature CHD (clinical CHD or sudden death documented in first-degree male relatives before age 55 or in first-degree female relatives before age 65) |
| Cigarette smoking (any cigarette smoking in the past month) |
| Hypertension (blood pressure |
| Low HDL-C (< 40 mg/dL)[a] |
| Risk Category | Initiate TLC | Consider Drug Therapy | LDC-C Goal |
|---|---|---|---|
| CHD and CHD risk equivalent | < 100 mg/dL | ||
| Noncoronary vascular disease | |||
| Type 2 diabetes | |||
| 10-year CHD risk > 20% | |||
| Multiple (2+) risk factors | |||
| 10-year CHD risk 10%-20% | < 130 mg/dL | ||
| 10-year CHD risk < 10% | < 130 mg/dL | ||
| 0-1 risk factor | < 160 mg/dL |
| Nutrient | Recommended Intake |
|---|---|
| Saturated fat[a] | < 7% of total calories |
| Polyunsaturated fat | Up to 10% of total calories |
| Monounsaturated fat | Up to 20% of total calories |
| Total fat | 25%-35% of total calories |
| Carbohydrate fiber | 50%-60% of total calories 20-30 grams per day |
| Protein | Approximately 15% of total calories |
| Cholesterol | < 200 mg/day |
| Total calories[b] | Adjust to maintain normal body weight |
| Drug Class, Agents, and Daily Doses | Average Lipid/Lipoprotein Effects | Adverse Effects | Contraindications | Clinical Trial Results |
|---|---|---|---|---|
| Bile acid resins[a] | LDL-C HDL-C TG -- no change or increase |
GI distress, constipation, decreased absorption of other drugs | Absolute: Dysbetalipoproteinemia G > 400 mg/dL Relative: TG > 200 mg/dL |
Reduced major coronary events and CHD death |
| HMG-CoA reductase inhibitors (statins)[b] | LDL-C HDL-C TG |
Myopathy, increased liver enzymes | Absolute: Active or chronic liver disease Relative: Concomitant use with certain drugs[c] |
Reduced major coronary events, CHD deaths, and total mortality |
| Nicotinic acid[d] | LDL-C HDL-C TG |
Flushing, hyperglycemia (or gout), upper GI distress, hepatotoxicity | Absolute: Chronic liver disease Severe gout Relative: Diabetes Hyperuricemia |
Reduced major coronary events, and possibly total mortality |
| Fibric acids[e] | LDL-C (may be increased in patients with high TG) HDL-C TG |
Dyspepsia, gallstones, myopathy | Absolute: Severe renal disease Severe hepatic disease |
Reduced major coronary events, increased non-CHD mortality (2 of 5 clinical trials) |
| [a]Cholestyramine (4-16 g), colestipol (5-20 g), colesevelam (2.6-3.8 g). |
| [b]Atorvastatin (10-80 mg), cerivastatin (0.3-0.8 mg), fluvastatin (20-80 mg), lovastatin (20-80 mg), pravastatin (20-40 mg), simvastatin (20-80 mg). |
| [c]Cyclosporine, gemfibrozil, macrolide antibiotics, various antifungal agents and cytochrome P450 inhibitors. |
| [d]Immediate-release (crystalline) nicotinic acid (1.5-3 g), extended-release nicotinic acid[] (1-2 g), sustained-release nicotinic acid (1-2 g). |
| [e]Gemfibrozil (600 mg twice daily), fenofibrate (200 mg), clofibrate (1,000 mg twice daily). |
| Parameter | Criteria |
|---|---|
| Waist circumference | Men: Women: |
| Triglyceride level | |
| HDL-C level | Men: < 40 mg/dL Women: < 50 mg/dL |
| Blood pressure | |
| Fasting glucose level |
| Risk Category | LDL-C Goal (mg/dL) | Non-HDL-C Goal (mg/dL) |
|---|---|---|
| CHD and CHD risk equivalent | < 100 | < 130 |
| Multiple ( |
< 130 | < 160 |
| 0-1 risk factor | < 160 | < 190 |
| Risk Factors | Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20-34 | 35-39 | 40-44 | 45-49 | 50-54 | 55-59 | 60-64 | 65-69 | 70-74 | 75-79 | |
| Age | -9 | -4 | 0 | 3 | 6 | 8 | 10 | 11 | 12 | 13 |
| Smoked any in past month, add: |
8 | 8 | 5 | 5 | 3 | 3 | 1 | 1 | 1 | 1 |
| Total cholesterol (mg/dL) | ||||||||||
| 160-199 | 4 | 4 | 3 | 3 | 2 | 2 | 1 | 1 | 0 | 0 |
| 200-239 | 7 | 7 | 5 | 5 | 3 | 3 | 1 | 1 | 0 | 0 |
| 240-279 | 9 | 9 | 6 | 6 | 4 | 4 | 2 | 2 | 1 | 1 |
| 11 | 11 | 8 | 8 | 5 | 5 | 3 | 3 | 1 | 1 | |
| HDL cholesterol (mg/dL) | ||||||||||
| -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | |
| 40-49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| <40 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Untreated systolic blood pressure (use this section only if the patient is not taking drugs for blood pressure) | ||||||||||
| 130-159 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Treated systolic blood pressure (use this section only if the patient is taking drugs for high blood pressure) | ||||||||||
| 120-129 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 130-159 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Score: | ||||||||||
| Risk Factors | Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20-34 | 35-39 | 40-44 | 45-49 | 50-54 | 55-59 | 60-64 | 65-69 | 70-74 | 75-79 | |
| Age | -7 | -3 | 0 | 3 | 6 | 8 | 10 | 12 | 14 | 16 |
| Smoked any in past month, add: |
9 | 9 | 7 | 7 | 4 | 4 | 2 | 2 | 1 | 1 |
| Total cholesterol (mg/dL) | ||||||||||
| 160-199 | 4 | 4 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 |
| 200-239 | 8 | 8 | 6 | 6 | 4 | 4 | 2 | 2 | 1 | 1 |
| 240-279 | 11 | 11 | 8 | 8 | 5 | 5 | 3 | 3 | 2 | 2 |
| 13 | 13 | 10 | 10 | 7 | 7 | 4 | 4 | 2 | 2 | |
| HDL cholesterol (mg/dL) | ||||||||||
| -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | -1 | |
| 40-49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| <40 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Untreated systolic blood pressure (use this section only if the patient is not taking drugs for blood pressure) | ||||||||||
| 120-129 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 130-139 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 140-159 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| Treated systolic blood pressure (use this section only if the patient is taking drugs for high blood pressure) | ||||||||||
| 120-129 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 130-139 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 140-159 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Score: | ||||||||||
| Men | Women | ||
|---|---|---|---|
| Point Total | 10-Year Risk % | Point Total | 10-Year Risk % |
| < 0 | < 1 | < 9 | < 1 |
| 0 | 1 | 9 | 1 |
| 1 | 1 | 10 | 1 |
| 2 | 1 | 11 | 1 |
| 3 | 1 | 12 | 1 |
| 4 | 1 | 13 | 2 |
| 5 | 2 | 14 | 2 |
| 6 | 2 | 15 | 3 |
| 7 | 3 | 16 | 4 |
| 8 | 4 | 17 | 5 |
| 9 | 5 | 18 | 6 |
| 10 | 6 | 19 | 8 |
| 11 | 8 | 20 | 11 |
| 12 | 10 | 21 | 14 |
| 13 | 12 | 22 | 17 |
| 14 | 16 | 23 | 22 |
| 15 | 20 | 24 | 27 |
| 16 | 25 | ||