
| Home |
| News 09/09/2544 |
| Clinical Significance of Antiretroviral Drug Levels Courtney V. Fletcher, PharmD. [Medscape HIV/AIDS: Annual Update 2001. © 2001 by iMedOptions, LLC.] |

Figure 1. Continuum of Antiretroviral TherapyEach box in Figure 1 highlights a source of variability that contributes to individual differences observed in the response to antiretroviral therapy. For example, a patient's adherence to the prescribed regimen directly affects the amount of drug that is available for absorption. The actual dose administered is also important; in antiretroviral therapy, there have been several cases in which suboptimal dosing regimens of potent agents have been demonstrated to be associated with worse outcomes (eg, the poorer results seen with saquinavir hard-gel vs saquinavir soft-gel, or with indinavir administered twice-daily vs 3-times-daily). The pharmacokinetic processes of absorption, distribution, metabolism, excretion, and protein-binding subsequently govern the amount of drug that is present in the systemic circulation and at the site of action. Considering drug metabolism solely, at least a 20- to greater than 1000-fold range in the amount of various cytochrome P450 isoforms has been demonstrated.[14,15] This provides a physiologic basis for variability in systemic concentrations for drugs that are substrates for cytochrome P450-catalyzed oxidative metabolism, such as HIV protease inhibitors (PIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs). PIs and NNRTIs are highly bound to plasma proteins, and differences in binding also contribute to variability in concentrations at the pharmacologic site of action; for example, the investigational protease inhibitor SC-52151 was orally absorbed and achieved plasma levels expected to be therapeutic, but its development was halted by the discovery that it was highly (70%) bound to alpha1 acid glycoprotein and plasma proteins, severely reducing cellular uptake, which explained the lack of any clinical anti-HIV effect.[16,17]
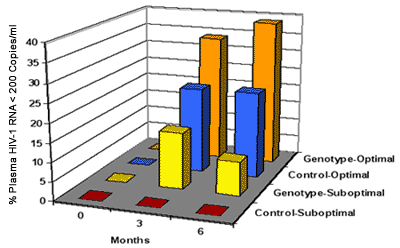
Figure 2. Proportions of Patients in the Viradapt Study Who Achieved Plasma HIV-1 RNA Level Less Than 200 Copies/mL, Analyzed By Study Arm and PI Concentrations*AIDS Clinical Trials Group (ACTG) study 359 compared the antiretroviral activity of 6 salvage regimens in subjects with plasma HIV-1 RNA levels between 2000 and 200,000 copies/mL who had received at least 6 months of prior therapy with indinavir.[39] A total of 277 individuals were randomized to receive saquinavir with ritonavir or nelfinavir, in addition to delavirdine, adefovir, or both. Participants were NNRTI- and adefovir-naive. Overall, one third of patients achieved virologic suppression to less than 500 copies/mL at week 16. Subjects who received delavirdine had a greater virologic response than did those who received adefovir (40% vs 18%). There was no difference between the delavirdine compared with delavirdine-plus-adefovir groups (40% vs 33%). This finding was surprising, as additive-to-synergistic activity was expected with the combination of delavirdine and adefovir, given the different mechanisms of action of these 2 drugs and given that subjects had not previously received either an NNRTI or adefovir. A companion study to ACTG 359, ACTG 884 was a pharmacokinetic evaluation of the concentrations of the agents in the 6 treatment regimens.[40] ACTG 884 found that saquinavir concentrations were 50% lower in the arms containing the combination of delavirdine plus adefovir compared with those receiving delavirdine alone. In addition, delavirdine concentrations were reduced by approximately 50% in the delavirdine-plus-adefovir arms compared with the delavirdine arms. These unexpected drug-drug interactions provide the most plausible explanation for the unexpected findings in ACTG 359 -- namely, why subjects in the delavirdine-plus-adefovir arm did no better than those who received delavirdine. As such, this study illustrates the clinical consequences of adverse drug-drug interactions that lower antiretroviral drug concentrations.
* Modified from Durant and colleagues[38]
Virologic Immunologic Pharmacologic
- Initial viral load
- Nadir of viral load reduction; time to nadir; decay rate constant
- Virus genotype and phenotype
- Cellular
- CD4+ function
- Cytotoxic T lymphocytes
- Humoral
- Chemokine
- CCR5
- CCR2b
- CXCR4
- Pharmacokinetic and pharmacodynamic properties
- Antiretroviral regimen
- Potency
- Simplicity
- Tolerance
- Optimal doses
- Adherence
Criteria Met/unmet for antiretrovirals? Pharmacologic Pharmacokinetic drug data are available Met Significant interpatient pharmacokinetic variability exists Met The drug has a narrow therapeutic index Met for some agents The pharmacologic effect observed persists for a relatively long period of time Met The pharmacologic effect is related to the drug concentration Met Clinical Clinical studies have documented the therapeutic range of the drug Unmet, although progress is being made with certain agents Plasma concentration reflects the concentration at the site of action Met Lack of effect is detrimental to the patient Met Analytical The drug assay is accurate, precise and specific, requires a small sample volume, yields results quickly, and is relatively inexpensive Met, although availability of antiretroviral assays is not widespread
| Oral Care and Dentinal Hypersensitivity W. Steven Pray, Ph.D., R.Ph., Professor of Nonprescription Products and Devices, School of Pharmacy, Southwestern Oklahoma State University, Weatherford, OK [U.S. Pharmacist 2609 2001. © 2001 Jobson Publishing Corp.] |
Help For Hypersensitive Teeth
Many people notice a painful sensation when they consume a food or beverage that is hot, cold, sweet or sour, or when brushing their teeth, or when a dentist cleans or dries the tooth with an air blast. The teeth may not hurt at any other time. This condition is dentinal hypersensitivity.
| Ultra-Low-Dose Oral Contraceptive Effectively Treats Acne |
| As a Class, Higher-Priced New Drugs Reduce Need for Hospitalization |